Anti-Borrelia and anti-cancer activity of cannabinoids
The first-line treatment of Lyme borreliosis (LB) is based on tetracycline antibiotics and β-lactam, as well as the cephalosporin antibiotics, however, it is effective only in the early stages when the latent persistent forms of Borrelia sp. have not appeared. Multiple or prolonged courses of antibiotics have been shown to be not beneficial in trials, and they can cause significant adverse effects.
In the search for alternative modes for the treatment of persistent forms of LB bacteria, the emphasis has been made on the categories of natural plant-derived compounds, with established therapeutic effects.

Cannabidiol (CBD) is one of the major hemp cannabinoids which has been given considerable attention due to its plethora of therapeutic properties and pharmacological activities such as analgesic, antibacterial, antidiabetic, antiemetic, antiepileptic, anti-inflammatory, antiproliferative, antipsychotic, antispasmodic, etc. Therefore, cannabinoids are considered as promising natural compounds for treating epilepsy, pain, depression, anorexia, and many other diseases and disorders.
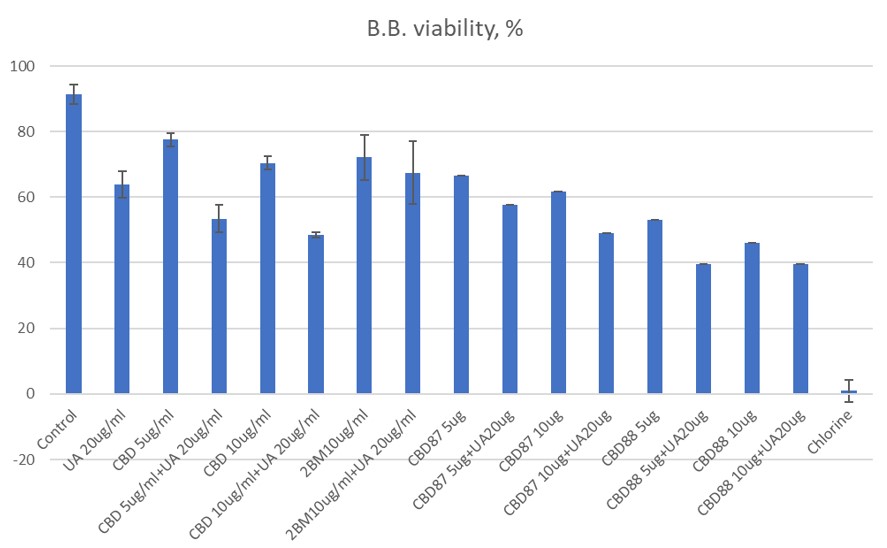
In the present study, we are testing several different industrial hemp products (evaporated (crude) hemp ethanolic extract, different CBD distillates, pure crystalline cannabidiol ≥99%) for their anti-borrelia and anti-cancer activities. For that we use borrelia bacterial cells (B. burgdorferi, strain 31) and cancer cell lines Huh7 (immortal human hepatic cell line), PC3 (prostate cancer cell line), LNCaP (human prostatic carcinoma), MCF7 (human breast cancer cell line), M21 (human melanoma cell line), SHSY5Y (neuroblastoma cell line) and normal cells HEK293 (immortalized human embryonic kidney cells).